What Would Happen if a Patient Hav a Continued Use of Cotisol and Glucocoticoids
Cortisol, the major glucocorticoid in humans, is essential to an individual's overall health. It also plays an important role in the body's response to stress. However, high and prolonged levels of cortisol, independent of classic Cushing syndrome due to adverse childhood experiences, have been linked to undesirable psychological and pathophysiological behaviors that sometimes continue into adulthood.
This article will review how the body controls cortisol secretion, how it is transported in plasma, its mechanism of action, as well as its pharmacology. We also discuss various laboratory measurements of cortisol and their appropriate use in diagnosing Cushing syndrome and cortisol deficiency.
Control of Cortisol Secretion
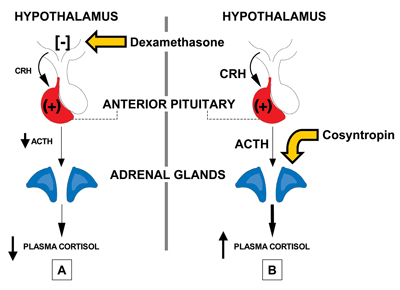
Cortisol is released from the adrenal cortex. The hypothalamus produces corticotrophin-releasing hormone (CRH), triggering the anterior pituitary to secrete adrenocortico-tropic hormone (ACTH), which in turn stimulates release of cortisol (Figure 1). CRH is transported to the anterior pituitary corticotrophs via the hypothalamic-pituitary portal system, and when it binds to specific receptors on the corticotrophs, this stimulates ACTH release into the bloodstream. Stress, physical activity, and low blood glucose concentrations prompt increased CRH release, while proinflammatory cytokines, such as IL-1, IL-6, and TNF-alpha, as well as vasopressin, also stimulate ACTH release.
In addition, cortisol levels are regulated by negative feedback loops in which the hormone reduces CRH secretion by the hypothalamus and reduces ACTH secretion by the anterior pituitary gland. The major site of negative feedback is the hypothalamus.
Cortisol Transport and Dynamics in Plasma
In blood, the majority of cortisol (80–90%) is attached to corticosteroid-binding globulin (CBG). The remainder is either loosely bound to albumin (7%) or unbound (2–3%).
Cortisol secretion has a marked diurnal rhythm that reflects diurnal CRH secretion. Concentrations are highest in the early morning and normally are lowest at midnight. Because of the extreme nature of this diurnal rhythm, labs should use separate reference intervals for morning and evening cortisol measurements. For example, the morning reference interval is typically 7–25 µg/dL, and the evening reference interval is 2–9 µg/dL, while midnight cortisol levels are <5 µg/dL.
Cortisol Action
Cortisol plays an important role in glucose homeostasis. It helps maintain normal levels of blood glucose by increasing the expression of gluconeogenic enzymes, such as glucose-6-phosphatase and phosphoenol-pyruvate carboxykinase, and it produces insulin resistance in skeletal muscle, adipose tissue, and the liver.
Cortisol stimulates glycogen synthesis through increased expression of glycogen synthase. Cortisol deficiency, however, can trigger hypoglycemia, and excess cortisol can produce diabetes mellitus. Cortisol also alters lipid metabolism, increasing adipose tissue lipolysis and raising circulating free fatty acid concentrations in plasma. Furthermore, excess concentrations of cortisol can cause hypertriglyceridemia and/or hypercholesterolemia.
When the body is under stress, cortisol plays a role in preventing hypotension. In high doses, glucocorticoids stimulate the strength of heart contractions, increase heart rate, maintain arteriolar tone, and decrease endothelial permeability. Cortisol also affects appetite, sleep, and behavior, with changes ranging from euphoria to depression and insomnia.
Cortisol also has potent anti-inflammatory and immunosuppressive effects. This is particularly apparent when it is administered as a drug at high dosages, but it also is important in the body's normal immune response. Consequently, glucocorticoids are widely used as drugs to treat inflammatory conditions such as arthritis and dermatitis, as well as autoimmune diseases.
Molecular Target of Cortisol
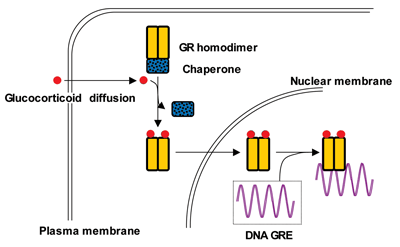
Cortisol's cellular target is a protein called the glucocorticoid receptor (GR), which is expressed on a variety of cells, including lymphocytes, macrophages, hepatocytes, and bone (Figure 2). After cortisol binds to the GR in the cell cytoplasm, the complex moves to the nucleus where it turns a variety of genes on or off via glucocorticoid response elements (GREs) in the promoters of the responsive genes. This interaction leads to increased or decreased mRNA synthesis depending upon the target gene. Evidence also suggests that GRs reside on the cell surface, which may account for the rapid effects of glucocorticoids.
Cortisol Pharmacology
A large number of glucocorticoids are available for clinical use, one of which is hydrocortisone, the pharmacological name for cortisol. In comparison to cortisol, these compounds vary in potency, duration of action, route of administration, and mineralocorticoid activity. Glucocorticoids may be administered orally, intravenously, intramuscularly, nasally, by inhalation, topically, or rectally. The oral and parenteral routes are the only ones intended for systemic absorption.
Nasal glucocorticoids are commonly used to treat allergic rhinitis, and patients often use inhaled glucocorticoids for bronchial asthma. Sometimes these administration routes can result in systemic absorption and cause local adverse effects. For example, oral or pharyngeal candidiasis (thrush) sometimes occurs in patients taking nasal or inhaled glucocorticoids.
Topical glucocorticoids are used to treat a large number of common skin disorders including eczema and psoriasis. Side effects include local atrophy of the skin. In some patients, inflammation or breakdown of the tissues allows greater systemic absorption. Rectal steroids are used to treat irritable colon or ulcerative colitis, where systemic absorption is also influenced by the state of the rectum.
Pharmacologically, glucocorticoids are classified as short-, intermediate-, or long-acting, depending on their half-life, typically <12 hours in the case of short-acting, ~18–36 hours for intermediate-acting, and 2–3 days for long-acting. Hydrocortisone and cortisone are examples of short-acting glucocorticoids. Hydrocortisone can be taken orally, administered parenterally, or used topically, whereas cortisone is taken orally. Prednisone, prednisolone, methylprednisolone, and triamcinolone are examples of intermediate-acting glucocorticoids. Dexamethasone is an example of a long-acting glucocorticoid. Excess glucocorticoids can produce varying elements of Cushing syndrome as a side effect.
Cortisol Measurements
Laboratories can measure total cortisol in plasma, serum, urine, or saliva, with immunoassays that are available from a number of manufacturers. Assay specificity, however, can be problematic as other steroids sometimes cross-react with cortisol. For example, in a recent version of one manufacturer's cortisol immunoassay, seven compounds had reported cross-reactivities of 4% or greater: 11-deoxycortisol (4.1%); corticosterone (5.8%); 21-desoxycortisol (45.4%); 6-betahydroxycortisol (158%); allotetrahydrocortisol (165%); 6-alpha-methylprednisolone (389%); and predniso-lone (171%). In contrast to prednisolone, which is the principle active metabolite of prednisone, cross-reactivity of the parent compound prednisone with cortisol is low (0.28%) and reminiscent of the low cross-reactivity of the cortisol immunoassay with dexamethasone (0.08%).
Laboratories can improve the specificity of immunoassays by extracting the cortisol from the patient's serum prior to assay; however, most laboratories do not perform this labor-intensive step.
Although excellent cortisol specificity can be achieved using mass spectroscopy (MS), turnaround time (TAT) suitable for clinical management of patients should be the same or next day. This type of TAT essentially requires an on-site MS facility, which only a limited number of institutions can support. Currently, MS is the only method capable of providing robust assays for free cortisol.
Laboratories also measure salivary cortisol. This assay is useful in screening for Cushing syndrome and requires excellent low-end sensitivity. As with free cortisol measurements, laboratories usually send out salivary cortisol specimens. The TAT is generally more liberal because immediate results are not necessary for patient management.
In addition to cortisol measurements, laboratories use immunoassays to measure ACTH. Because ACTH is very labile, care should be taken to draw and process samples correctly. CRH is not usually measured in suspected cases of cortisol excess or deficiency.
Potential Glucocorticoid Excess
Physicians order laboratory tests when cortisol excess or deficiency is a clinical possibility. Cortisol excess produces the clinical phenotype of Cushing syndrome, which includes a wide range of physical findings such as weight gain, centripetal obesity, moon facies, buffalo hump, stria, osteoporosis, collapsed vertebrae, pathologic fractures, poor growth in children, cataracts, poor wound healing, gastric ulcer, hypertrichosis, hirsutism, acne, and hypertension. Patients also may exhibit psychosis, increased appetite, or increased susceptibility to infection. Laboratory findings include hypokalemia, alkalosis, hyperglycemia and/or low eosinophil count. Frequently, these findings can be attributed to glucocorticoids used to treat inflammatory or autoimmune diseases. In such cases, laboratory testing is not indicated.
In the absence of exogenous glucocorticoid administration, physicians should order laboratory testing to determine the cause of the symptoms. While the complete evaluation of a patient for diagnosis of Cushing syndrome is beyond the scope of this article, it is worthwhile reviewing the three screening tests for hypercortisolism.
Measuring cortisol excretion in a 24-hour urine collection, known as urinary free cortisol (UFC), provides an integrated measurement of cortisol production. To evaluate the sample for completeness of collection, urine creatinine also should be measured. In true exogenous Cushing syndrome, UFC is usually 2–3 times the upper limit of the reference interval.
Another screening method for hypercortisolism involves overnight dexamethasone suppression of cortisol (Figure 1A). For this test, adults receive 1 mg of dexamethasone orally at 10 p.m. and have blood drawn for the cortisol measurement at 8 a.m. the next morning. Normally, this early-morning cortisol level is <5 µg/dL, and ≥10 µg/dL is considered abnormal. Cushing syndrome is not excluded if the level is 5–10 µg/dL.
A third way to rule out cortisol excess is to measure cortisol from a blood sample drawn at midnight when levels are normally at their lowest, <5 µg/dL. However, since arranging for a venipuncture on a patient sleeping at home is extremely difficult, the alternative approach is for the patient to obtain a saliva sample at midnight. The next morning, the saliva can be delivered to the laboratory for send-out analysis. Proper sample collection, however, is critical for obtaining a valid result, and getting patients to precisely follow instructions may be difficult. Patients should not eat food for 60 minutes prior to obtaining the sample, and they must not brush or floss their teeth immediately prior to testing to avoid contamination of the saliva with blood. They also need to wash out their mouth thoroughly with water 10 minutes before specimen collection.
Patients who have an abnormal initial screening test should be tested again to confirm the diagnosis, with either a repeat of the first test or one of the other screening tests. If the results of two screens are in conflict, normally labs will perform a tie-breaker screening test. Cushing syndrome diagnosis requires that two of two or two of the three screening tests be abnormal and indicate hypercortisolism.
Potential Glucocorticoid Deficiency
Cortisol deficiency can be classified as primary, resulting from adrenal gland failure—known as Addison disease—or secondary, resulting from ACTH, or tertiary, resulting from CRH deficiency. Together, ACTH or CRH deficiency can be described as central adrenal insufficiency. Significantly, aldosterone deficiency is also usually present in cases of primary adrenal insufficiency. Cases of central adrenal insufficiency are usually not as severe as primary adrenal insufficiency because in the former, aldosterone is not deficient.
Neither primary nor central endogenous adrenal insufficiencies are common. However, immediate withdrawal of glucocorticoid replacement therapy can trigger cortisol insufficiency or even adrenal crisis. Adrenal crisis is the clinical syndrome of hypotension, hypoglycemia, hyponatremia, acidosis, and obtundation from acute glucocorticoid deficiency. Patients who also exhibit mineralocorticoid deficiency are considered to have Addisonian crisis, which typically is a more severe clinical phenotype than isolated acute glucocorticoid deficiency. In Addisonian crisis, hyperkalemia may also be observed. Untreated adrenal crisis can be fatal.
The laboratory findings for the various states of cortisol deficiency can include hyponatremia, hyperkalemia if aldosterone is deficient, acidosis, hypoglycemia, and/or eosinophilia. The best way to test for cortisol deficiency is to measure this analyte at the time of an adrenal crisis, such as during stress, when it normally is ≥20 µg/dL. In the absence of crisis, a 250-µg dose of cosyntropin, a synthetic polypeptide with ACTH activity, can be administered intravenously or intramuscularly after an overnight fast (Figure 1B). The patient's blood is drawn for testing before the dose for a baseline level and again at 30 and 60 minutes. If only a single stimulated sample can be drawn, it should be taken at 45 minutes. A normal response to cosyntropin is a peak cortisol of ≥20 µg/dL.
Investigators have sought to determine if a lower dose of cosyntropin (1 µg versus 250 µg) in the 1-hour stimulation test might be more sensitive for detecting adrenal insufficiency from either ACTH deficiency or primary adrenal failure. Some patients who have normal responses to 250 µg of ACTH have an inadequate response to 1 µg. This dosage change remains controversial, and currently the 1-µg ACTH dose has not been accepted as superior for detecting glucocorticoid deficiency.
More invasive tests of cortisol secretion involve insulin-induced hypoglycemia and metyrapone testing procedures. Insulin-tolerance tests (ITTs) are not typically used as the first-line investigation for cortisol deficiency because of the acute neurologic risk of hypoglycemia and the sustained need for good intravenous access throughout the test. Metapyrone blocks cortisol synthesis from 11-desoxycortisol. In normal individuals, decreased cortisol levels will elicit an increase in ACTH secretion, producing an accumulation of 11-desoxycortisol.
This effect can be measured as an increased level of the urinary metabolite, 17-hydroxycorticosteroids, or an increase in the ratio of 11-desoxycortisol to cortisol. Like ITTs, metapyrone testing is not a first-line test.
In the developed world, most cases of Addison disease result from autoimmune destruction of the adrenal gland. Adrenal autoantibodies can be sought by indirect immunofluorescence or by testing for autoantibodies to CYP21. Autoimmune Addison disease can be associated with other immunoendocrinopathies in the autoimmune polyglandular syndromes types 1 and 2.
In any of the investigations described above, primary adrenal insufficiency and central adrenal insufficiency can not be distinguished if cortisol is deficient; therefore, additional testing is necessary to make a diagnosis.
Appropriate Testing
Disorders involving either excess or deficient cortisol levels are serious and can be life-threatening when left untreated. Random cortisol measurements are usually not diagnostically helpful; therefore, laboratorians need to work closely with physicians to ensure appropriate tests are ordered. Useful diagnostic information for cortisol requires testing under specific, controlled conditions.
Like many other hormones, deficiency states are best assessed by measuring cortisol in stress states or after pharmacologic stimulation. In cases of suspected endogenous hypercortisolism, interaction between clinicians and laboratorians is essential to assess patients with a suspected perturbation of cortisol.
SUGGESTED READING
Bertholf RL, Jialal I, Winter WE. The adrenal cortex. In: Burtis C, Ashwood E, Bruns D, eds. Tietz textbook of clinical chemistry and molecular diagnostics. 5th Ed. St. Louis: Elsevier Saunders 2012:1847–904.
Cohen J, Pretorius C, Venkatesh B. Dismissal of the utility of free cortisol measurement is premature. Intensive Care Med 2012;38:718.
Haller MJ, Winter WE, Schatz DA. Autoimmune polyglandular syndromes. In: Sperling M, ed. Pediatric endocrinology. 3rd Ed. Philadelphia: W.B. Saunders 2008:770–87.
Winter WE, Hardt NS, Harris NS. Disorders of the adrenal cortex and medulla. In: Clarke WA, Dufour DR, eds. Contemporary practice in clinical chemistry. 2nd Ed. Washington, DC: AACC Press 2011:451–70.



Source: https://www.aacc.org/cln/articles/2012/september/cortisol
0 Response to "What Would Happen if a Patient Hav a Continued Use of Cotisol and Glucocoticoids"
Post a Comment